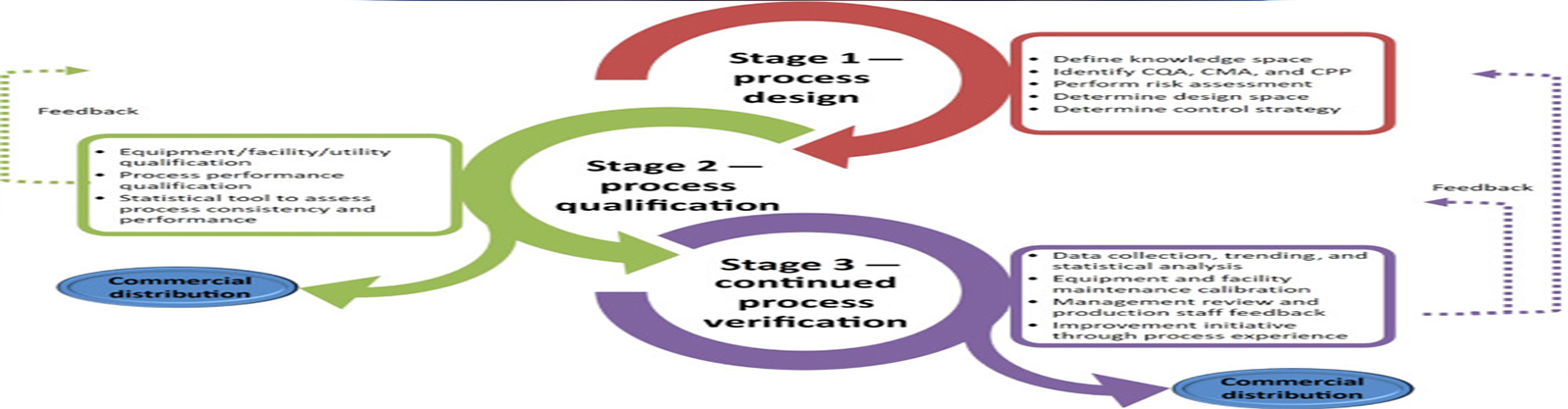
Qualifications & Validations
We provide guidance & prepare Qualification & Validation (Equipment, Instrument & Utilities etc) documents for followings:
- Pharmaceutical Formulations/Drug Products Manufacturing Facility Tablets, Capsules, Liquid Orals, Injections & Ointment)
- Food Products manufacturing facility
- Medical Devices Manufacturing facility
- API manufacturing facility (Sterile & Non-Sterile)
- Key Starting Material and Intermediate Manufacturing facility
- Contract research organizations/laboratories
Qualifications
- Facility
- Equipment (URS, DQ, IQ, OQ & PQ)
- HVAC System
- Water generation and distribution System
- Compressed Air & Nitrogen System
- Heat Mapping Studies.
Validations
- Process Validation
- Cleaning Validation
- Analytical Method Validation
- Media Fill Validation
- Computer System Validation